Bird influenza is sent generally by wild birds, like these snow geese. The H5N1 bird influenza infection is triggering the biggest bird influenza break out in history, contaminating countless birds and a growing series of mammal types. Though human infection is presently unusual, effective transmission in between farmed minks in Spain raises issues about prospective human transmission. Simply a couple of anomalies might make H5N1 more effective at contaminating people.
A break out of H5N1 bird influenza that began in 2021 has actually ended up being the biggest bird influenza break out in history, both in the U.S. and worldwide. In the U.S. the infection has actually resulted in the damage of countless commercially raised chickens, turkeys, ducks, and geese, and has actually eliminated countless wild birds.
Numerous virologists are worried that this infection might overflow to people and trigger a brand-new human pandemic. University of Colorado Stone virologists Sara Sawyer, Emma Worden-Sapper and Sharon Wu sum up the engaging story of H5N1 and why researchers are carefully viewing the break out.
1. Is this infection a severe danger to people?
H5N1 is a particular kind of influenza infection, primarily harbored by birds, that was initially spotted on a goose farm in China in 1996 Just recently it has actually started contaminating a blowing up variety of bird and mammalian types around the world.
Fortunately about H5N1 for people is that it presently does not spread out well in between individuals. The majority of people who have actually contracted H5N1 have actually gotten it straight from connecting with contaminated poultry — particularly chickens, turkeys, ducks and geese, which frequently are raised in close quarters on big business farms.
There are just a little handful of examples of human-to-human spread. Since H5N1 does not spread out well in between individuals, and since direct infection of people by contaminated birds is still fairly unusual, H5N1 has not yet emerged into a human epidemic or pandemic.
2. Why is this break out unexpectedly getting a lot attention?
The very first factor that a lot attention is being paid to bird influenza today is that presently H5N1 is triggering the biggest “bird pandemic” ever tape-recorded A specific viral version that emerged in 2020, called H5N1 2.3.4.4 b, is driving this break out.
In farming poultry flocks, if a couple of birds test favorable for H5N1, the entire flock is eliminated despite signs or infection status. Greater costs for eggs and poultry meat in the U.S. are one outcome. The Biden administration is thinking about immunizing farmed poultry flocks, however the logistics might be rather made complex.
The 2nd factor for increased attention is that H5N1 is now contaminating more bird and mammalian types than ever in the past. The infection has actually been spotted in a broad selection of wild birds and in varied mammals, consisting of badgers, black bears, bobcats, coyotes, ferrets, fisher felines, foxes, leopards, opossums, pigs, skunks and sea lions.
As H5N1 contaminates more types, it likewise increases its geographical variety and produces more viral variations that might have brand-new biological residential or commercial properties.

Peru decreed a 90-day health emergency situation in December 2022 after more than 13,000 pelicans passed away on its beaches, potentially contaminated with H5N1
The 3rd and most uneasy factor that this infection is getting a lot press is that H5N1 now appears to be sending well in between people of a minimum of one mammalian types. In late 2022, mammal-to-mammal spread happened in Spain in farmed minks H5N1 spread extremely effectively in between the minks and triggered scientific indications of health problem and death in the mink populations where it was spotted.
Sea lions in Peru are likewise catching H5N1 infection in huge numbers. It hasn’t been validated definitively whether the sea lions are spreading out the infection to each other or are contracting it from birds or H5N1-infected water.
Here’s the essential concern: If H5N1 can attain spread in minks and potentially sea lions, why not people? We are likewise mammals. It holds true that the farmed minks were restricted in close quarters, like chickens on a poultry farm, so that might have contributed. However people likewise reside in high densities in lots of cities around the globe, offering the infection comparable tinder need to a human-compatible alternative develop.
The World Health Company is carefully keeping track of and examining the spread of H5N1 in mammals.
3. What functions could assist H5N1 spread well in people?
Birds experience influenza as an intestinal infection and spread influenza primarily through defecating in water By contrast, people experience influenza as a breathing infection and spread it by breathing and coughing.
Over the centuries, a few of these bird influenza infections have actually been passed from birds to people and other mammalian types, although this is a fairly unusual occasion.
This is since bird influenza infections should alter in numerous methods to contaminate mammals effectively The most essential mutational modifications impact the tissue tropism of the infection– its capability to contaminate a particular part of the body.
Bird influenza infections have actually progressed to contaminate cells of the intestinal tract, while human influenza infections have actually progressed to contaminate cells of the breathing system. Nevertheless, in some cases an influenza infection can obtain anomalies that enable it to contaminate cells in a various part of the body.
Which cells influenza contaminates is partly determined by the particular receptor that it binds. Receptors are the particles on the surface area of host cells that an infection makes use of to go into those cells. As soon as infections remain in cells, they might have the ability to produce copies of themselves, at which point an infection has actually been attained.
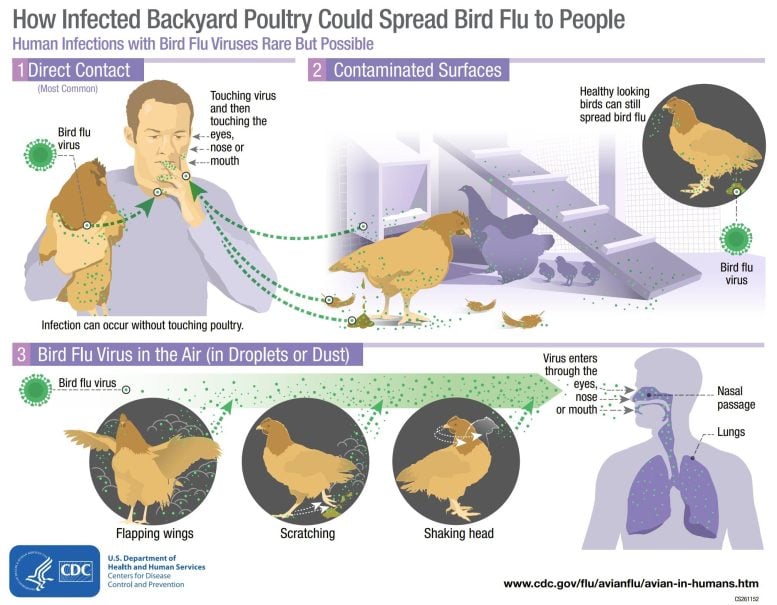
Bird influenza infections in individuals are unusual, however possible. The majority of reported bird influenza infections in individuals have actually taken place after vulnerable contact with contaminated birds or infected surface areas. Credit: United States CDC
Both human and bird influenza infections utilize receptors called sialic acids that prevail on the surface areas of cells. Bird influenza infections, such as H5N1, utilize a variation called a2,3-linked sialic acid, while human influenza infections utilize a2,6-linked sialic acid– the primary version in the human upper breathing system. Hence, to end up being effective at contaminating people, H5N1 would likely require to alter to utilize a2,6-linked sialic acid as its receptor.
This is an issue since research studies have actually revealed that just a couple of anomalies in the viral genome suffice to change receptor binding from a2,3-linked sialic acid to the human a2,6-linked sialic acid. That does not look like much of a hereditary challenge.
4. Why do not we make a vaccine simply in case?
With bird influenza infections, it is not possible to make reliable human vaccines beforehand, since we do not understand precisely what the genes of the infection will be if it begins to spread out well in people. Bear in mind that the seasonal influenza vaccine should be remade every year, although the basic kinds of influenza infections that it secures versus are the very same, since the particular hereditary variations that impact people alter from year to year.
Today, the very best method individuals can safeguard themselves from H5N1 is to prevent contact with contaminated birds. To learn more about avoidance, particularly for individuals who keep domesticated birds or are bird-watching enthusiasts, the Centers for Illness Control has a list of standards for preventing H5N1 and other bird influenza infections
Composed by:
- Sara Sawyer, Teacher of Molecular, Cellular and Developmental Biology, University of Colorado Stone
- Emma Worden-Sapper, PhD Trainee in Molecular, Cellular and Developmental Biology, University of Colorado Stone
- Sharon Wu, PhD Trainee in Interdisciplinary Quantitative Biology and Molecular, Cellular, and Developmental Biology, University of Colorado Stone
This short article was very first released in The Discussion